Medical Device Industry Services
General Medical Device Services

Safe reusable medical devices

Biological evaluation report
We provide you with everything you need to plan, test, and document compliance with ISO 10993-1. This is a crucial step toward securing a CE Mark certificate and getting your medical device to market in the EU and beyond.
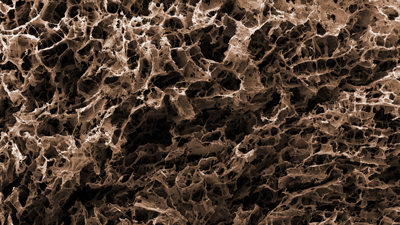
Surface characterisation
Our analytical expertise helps get your medical devices through the physico-chemical, morphological, and topographical (PMT) characterisation process and ensure compliance with ISO 10993-19.

Timely materials screening
We can help you select and evaluate your medical devices construction materials for suitability and compliance readiness with regulatory requirements.

Manage change and stay safe

QMS for medical device manufacturers
Device Specific Services

Safe devices for drug products
We provide you with everything you need to test and document device compatibility with drug products in compliance with regulatory requirements.

Breathing gas pathways
We can help you plan, test, and document compliance with regulatory requirements and get your breathing gas pathway products ready for CE Mark certification.

Safe in-vitro diagnostics
We help you ensure that your in-vitro diagnostic medical device complies with (EU) 2017/746 (IVDR) Annex I and is ready to be certified with a CE Mark.

Safe stents and implants
We can help you get your stents and implants ready for CE Mark certification with our expert understanding of the ISO 10993-1 standard.