Latest News
Medical Implant Biosafety
Ensuring the biosafety of your medical implants is necessary for getting them in compliance with relevant regulations.
Medical implants come into intimate contact with the human body for periods lasting from a few hours to permanently. As such, they are subject to strict regulations regarding their safety. Manufacturers of medical implants need to apply for a CE Mark certificate if they are to be sold and used in the EU. A CE certificate verifies that a product is safe and performs as intended. The ISO 10993-1 standard describes the requirements for evaluating and testing the biological safety of all constituent materials in a medical device product.
SAXOCON has the expertise to help you get your implants compliant and certified for sale in the EU.
Read more about our services here.
Workplace Air Quality
Are you a manufacturer? Do you know what particles your workers are exposed to in your working environment? Whether it’s additive manufacturing, assembly, welding or any other process that produces dust or fumes, your workers are being exposed to particles. Mitigating any health concerns for your workers requires understanding how many and the nature of the particles produced. Usually, this understanding requires long-term comprehensive studies to assess potential toxicological exposure.
SAXOCON can help you quickly understand the potential risks involved in your manufacturing processes by coming to you and, using our novel tool, the SAXOCON Impactor, sampling particles from the air in your workplace directly onto electron microscopy grids. This process greatly accelerates the assessment process so you and your workers can have peace of mind.
Check out how we can help here.
Reusable Medical Device Safety
Do you manufacture reusable medical devices?
If so, your products are likely used in medical facilities around the world.
In the EU, manufacturers wanting to sell reusable medical devices must ensure that their cleaning, disinfection, and sterilisation procedures are validated.
SAXOCON has the expertise to help you get your procedures into compliance with all relevant guidelines, such as AAMI TIR12 and ISO 17665, and keep your products on the market or get your new products approved quickly.
White Paper – Medical Devices and Particulates
Medical devices are often vital for a patient’s well-being and must therefore be safe and should definitely not worsen their condition. However, there is a high risk of particulate contamination for intravenous and infusion medical devices that can cause health issues such as inflammation, irritation or even blood clots.
Several factors determine the level of risk a particle presents, with particle size, shape, and composition determining the associated health risks. Once found, particles can be analysed using automated microscopy, computational counting methods, and surface analysis of their physical, topographical, and morphological properties.
According to international standards, these analyses should be performed using an, at minimum, ISO Class N5 certified laboratory to minimise potential contamination. Likewise, it is necessary to conduct these analyses with the utmost precision and concentration.
SAXOCON offers a tailor-made analysis for your medical devices with a clean ISO Class N2 laboratory – which is an even higher standard than the minimum required ISO Class N5. We conduct all required tests following ISO 8536-4, USP 788, and other relevant guidelines. Furthermore, SAXOCON conducts a toxicity analysis of any particles found in your products.
By filling out the form below, you can download our white paper about medical devices and particulates and the consequences for your testing and manufacturing processes.
EFSA Deadline for Food Enzyme Documentation Fast Approaching
Are you ready for the upcoming requirements for submission and approval of your food enzymes?
In March 2020, EFSA announced changes in how food enzymes compiled in joint dossiers will be evaluated. The deadline for meeting the new requirements is 21 April 2022. If you currently have included your food enzymes in a joint dossier, you will need to conduct an updated toxicological study to ensure compliance. SAXOCON’s highly skilled toxicologists can help you plan for the upcoming deadline and test and document the safety of your food enzymes.
Joint dossiers create several challenges and data gaps in currently submitted documentation, which is why EFSA is now requiring that enzyme applicants send a company-specific comprehensive data package. The updated requirements require conducting a toxicological assessment in accordance with regulatory guidelines.
Until now, it has been possible for the producers of food enzymes to group individual food enzymes under one umbrella application, provided the enzymes had the same catalytic activities, were manufactured using a similar process, and originated from the same organism. However, due to confidentiality issues, the current procedure has led to a lack of specific information regarding aspects like the production strain or the chemical composition of each food enzyme.
EFSA provides detailed information about these changes in this article.
SAXOCON can help you prepare for these regulatory changes.
Read more about how we can help here.
Chemically Non-compliant Products Sold Online in EU
A recent report by the ECHA found that over half the products sold online in the EU were non-compliant with one or more EU requirements with regard to chemicals. These findings resulted in more than 5000 enforcement actions by national authorities.
As a product manufacturer, this is an entirely avoidable problem. With the proper understanding and traceability of the chemicals present in your products and the suppliers of these materials, you can avoid running afoul of regulators.
Don’t get caught with your chemical pants down at the next inspection. SAXOCON has the expertise to help you ensure that your products are compliant with all relevant regulations.
Nitrosamine Impurities
Are you a pharmaceutical manufacturer? Do you know whether your products contain nitrosamine impurities and, if so, whether they are below the thresholds established by the various regulatory agencies?
SAXOCON has the expertise and services necessary to help you to fill in the gaps in your supply chain information and assess the impact on your drug substances and products.
Nitrosamines are highly toxic compounds. Some are considered to be even more carcinogenic than currently established thresholds and are subject to strict regulations in both the EU and the US.
Check out how we can help here.
SAXOCON at the COMPAMED/MEDICA Trade Fair 2021
SAXOCON is participating at the COMPAMED/MEDICA trade fair in Düsseldorf, we will be there on November 15-17. We will have a booth at the Danish Pavilion. Come and meet us there.
Click here for more information.
Reduce Animal Testing
Every day people are exposed to chemicals from a variety of sources such as food products, cosmetics, clothing, medical devices, and medicines. EU regulations set requirements for testing chemicals for their potential to cause harm, be it through DNA damage, cancer, hormonal disruption or some other way.
Often the testing for the harmful effects of a chemical is conducted through testing on animals. While effective, animal testing suffers from grave ethical concerns around the treatment of animals. On 29 September this year, the European Medicines Agency (EMA) implemented new measures to minimise the use of animal testing in the development of medicines.
Achieving the goal of reduced animal testing requires the use of sophisticated computer modelling and the expertise to interpret the results. SAXOCON specialises in the use of Quantitative Structure-Activity Relationship (QSAR) modelling and can therefore help you to understand the potential danger of the chemicals used in your products without animal testing.
So, get compliant with EU regulations and minimise the use of animal testing with SAXOCON’s QSAR specialists.
Read more about how we can help here.
Surface Characterisation
Surfaces are everywhere, and we constantly come into contact with them. To live in this world means touching surfaces, so we rarely give it any thought. But, the things we manufacture, the things we wear on or have implanted in our bodies that we come into contact with, must be safe.
Any product marketed in the EU that comes into direct contact with skin, blood or organs; or has indirect contact via packaging or conducting air or fluids that interact with people must be CE Certified. Depending on the duration and type of body contact a product has, it must undergo a defined physicochemical, morphological, and topographical (PMT) analysis.
Correctly conducting a PMT analysis requires care and skill and is a critical step in ensuring product safety. Once completed, you will be better prepared to make well-informed decisions about your product and its readiness for the market.
SAXOCON has the expertise to help you get your products compliant and certified for sale in the EU.
Check out how we can help here.
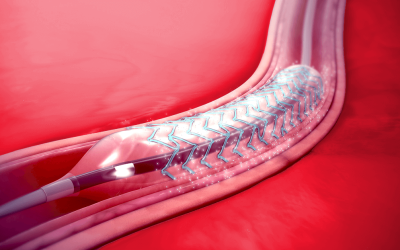
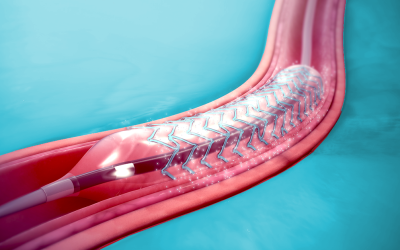
Compliant Stents
Get your stents and implants in compliance with relevant regulations.
Stents and other medical implants come into intimate contact with the human body, sometimes over long periods or permanently. As such, they are subject to strict regulations regarding their safety. If you are a manufacturer of stents or implants and want to market them in the EU, you will need to apply for a CE Mark certificate. A CE certificate verifies that a product is safe and performs as intended. The ISO 10993-1 standard describes the requirements for evaluating and testing the biological safety of a medical device product, including physicochemical, morphological characteristics, and topographical characterization of constituent materials.
SAXOCON has the expertise to help you get your stents and implants compliant and certified for sale in the EU.
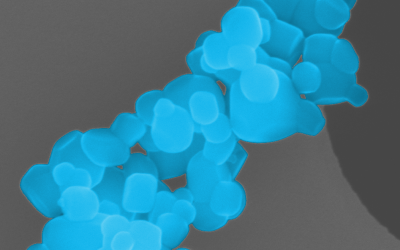
Nanomaterials in Your Products
Do you use nanomaterials in your production processes, or are they in your final finished product? If so, you are subject to EU regulations that require you to know and document your use of nanomaterials.
According to ECHA, products incorporating or consisting of nanomaterials are subject to REACH and CLP regulations. Manufacturers, suppliers, and importers must be able to identify the use of nanomaterials in their products to ensure that they meet the requirements of these regulations.
SAXOCON has the tools and expertise to identify and characterise any nanomaterials in your products. We provide you with the required documentation to get your product approved for sale in the EU.
Check out how we can help here.