Latest News
Material Readiness
Whether you are manufacturing medical devices, pharmaceuticals or combination products, you will be using materials. Marketing your products in the EU requires that you ensure the quality and safety of the materials used in your products. Early and robust material selection is key to meeting regulatory requirements and mitigating the risk of unexpected events.
In our latest webinar, SAXOCON CTO, Carsten B. Senholt, discusses the importance of materials readiness and its role in the proper selection of robust materials candidates for production.
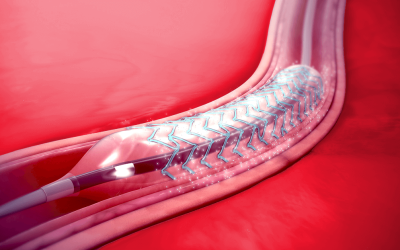
Safe Stents and Implants
Stents and other medical implants are products that have a high level of contact with the human body. As such, they are subject to strict regulations regarding their safety. If you are a manufacturer of stents or implants and want to market them in the EU, you will need to apply for a CE Mark certificate. This certificate verifies that the product is safe and performs as intended. The ISO 10993-1 standard prescribes a process for evaluating and testing the biological safety of a medical device product, including the physicochemical, morphological, and topographical characterization of materials.
SAXOCON has the necessary expertise to help you get your stents and implants compliant and certified for sale in the EU.
Check out how we can help here.
Safe Reusable Medical Devices
Reusable medical devices are ubiquitous and necessary tools for medical facilities around the world. As a manufacturer of reusable medical devices who wants to sell them in the EU, you will need to ensure that your cleaning, disinfection, and sterilisation procedures are validated.
SAXOCON has the expertise to help you get into compliance with all relevant guidelines, such as AAMI TIR12 and ISO 17665, and keep your products on the market or get your new products approved in a timely manner.
Nanomaterials White Paper
Download our white paper on nanomaterials today!
The new EU Medical Devices Regulation (MDR) and IVD Regulation (IVDR) do include specific classification rules for devices that consist of or incorporate nanomaterials. For medical device manufacturers, it is important that they can identify the use of nanomaterials in their devices to ensure that they meet the requirements of these new regulations.
At SAXOCON we have the expertise and tools for identifying and characterising nanomaterials and providing you with the required documentation for your final finished device.
Fill out the form below to read about their consequences for regulations and your manufacturing process.
Surface Characterisation
Surfaces are everywhere, and we constantly come into contact with them. To live in this world means touching surfaces, so we rarely give it any thought. But the things that we manufacture that we come into contact with must be safe. This is true for everything we use or wear but especially so for medical devices.
Any medical device that comes into direct contact with people’s skin, blood or organs; or has indirect contact via contact with food or by conducting air or fluids that interact with people must be CE Certified to be marketed in the EU. Depending on their duration and type of body contact must undergo a physicochemical, morphological, and topographical (PMT) analysis, as defined by ISO 10993-19.
Correctly conducting a PMT analysis requires care and skill and is a critical step in ensuring product safety. Once completed, you will be better prepared to make well-informed decisions about your medical device and its readiness for the market.
SAXOCON has the expertise to help you get your medical devices compliant and certified for sale in the EU.
Check out how we can help here.
Indoor Air Quality Investigations
Air quality problems can often be difficult to pinpoint? You can be doing everything by the book concerning air temperature, humidity, and CO2 levels and still have an air-quality problem. In most cases, when confronted with persistent and difficult-to-diagnose, air-quality mysteries, the culprit is Ultra Fine Particles (UFP).
UFPs are defined as those particles with a diameter of less than 0.1 micrometres. Such particles make up the vast majority of particles found but only account for a fraction of the present mass.
UFPs are usually the by-products of combustion or chemical reactions, such as:
- Laser printers
- Cleaning agents and chemical storage
- Kitchen usage and smoking areas
- Boiler and furnace leaks
- HVAC duct and filter leaks
- Grinding, welding, soldering, and powder handling
- Vehicle emissions and outdoor air pollutants sources
Due to their small size, UFPs often travel along unexpected pathways to areas that may be far from their source.
SAXOCON can help you find the emitting source(s) and to control or even remove them.
Check out how we can help you here.
Mind the Gap on Nitrosamine Impurities
March 31st is the deadline for the completing your step 1 risk evaluation on potential nitrosamine impurities in medical products. These highly toxic compounds, some of which are considered to be even more carcinogenic than currently established thresholds, are subject to strict regulation in both the EU and US.
Let SAXOCON help you to fill in any gaps in your supply chain information and assess their impact on your drug substances and products.
Check out how we can help here.
Webinar: Pharmaceutical Manufacturing Construction Materials – Part 1
Are your production equipment and systems qualified for product contact?
On Wednesday 13 January 2021 from 15:00 – 16:30, we will be hosting a webinar on the role and design of extraction studies to ensure safety and quality in pharmaceutical manufacture.
Programme:
- Welcome and introduction to presenters
- How to meet regulatory expectations for risk assessment of E&L in single-use systems – with Carsten Baun Senholt, Chief Technical Officer, SAXOCON A/S
- BioPhorum Operational Group E&L for single-use systems – The final chapter – with Carsten Worsøe, Principal Scientist Extractables and Leachables, Novo Nordisk
- Q & A from the chat window
SAXOCON CTO at the Extractables and Leachables Forum 2021
SAXOCON is pleased to announce that our CTO, Carsten B. Senholt, will present at the 2021 Extratables and Leachables forum.
Carsten will discuss the importance of materials readiness and its role in the proper selection of robust materials candidates for production.
Proper materials selection is key to an intelligent and cost-effective extractables and leachables test strategy.
More information about the forum can be found here.
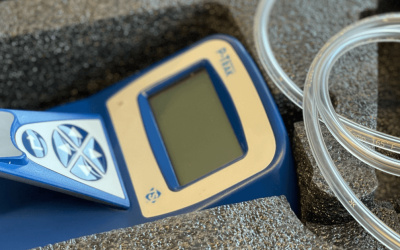
Secure Worker Health and Safety
Do you know what particles your workers are exposed to in your working environment? Which of these particles is a concern for the health of your workers? Usually, understanding the risks posed by particles associated with processes that generate dust or involve handling powder requires long-term comprehensive studies to assess potential toxicological exposure.
Our novel tool, the SAXOCON Impactor, quickly collects particles from the air in your workplace directly onto electron microscopy grids, which allows us to accelerate the assessment process.
Check out how we can help here.
Webinar: Pharmaceutical Manufacturing Construction Materials – Part 2
Join us for part 2 of our webinar series on pharmaceutical manufacturing construction materials on Wednesday 3 February 2021 from 14:00 – 15:30
In this seminar, we will discuss how to address particulate matter findings in pharma production facilities.
Programme:
- Welcome and introduction to presenters
- Is particulate matter a safety concern? – by Carsten Baun Senholt, Chief Technical Officer
- Fast-track physicochemical analysis – by Kirsten Inga Kling, Senior Scientist Analysis and Characterisation
- Q&A from the chat window
Did You Skip SCIP?
As of today, 5 January 2021, all suppliers of physical products in the EU market are required to track and report the content of substances of very high concern (SVHC). This information shall be made available to consumers and waste operators by filling in the Substance of Concern in Products (SCIP) with the European Chemical Agency (ECHA). https://echa.europa.eu/scip
SAXOCON can help you comply with your obligations for tracking your supply chain, evaluating potential SVHC, and submitting your information to ECHA.
Check out how we can help here.
 Medical Devices
Medical Devices Pharmaceuticals
Pharmaceuticals Chemicals
Chemicals Analytics Services
Analytics Services Food Industry
Food Industry Cosmetics
Cosmetics